We like DNA… a lot. It holds our genetic information; the heritable part of us that we receive from our forbearers and pass on to our young. But DNA is under attack. Every day a whopping 100,000 or so lesions form in our DNA in just one cell. This is not a problem, most of the time, since the cell is equipped with its own DNA damage response (DDR) that can repair any breaks or changes in the DNA sequence. Key to this response is the ‘guardian of the genome’, p53. Not a very exciting name, but what it lacks in appellation, it makes up for in awesomeness. When there is damage, p53 comes along to save the day, interacting with and regulating the expression of a whole host of other proteins and RNAs in the cell. A bit of damage is easy work for p53, but when DNA mishaps are too frequent or too severe to deal with, p53 may instead induce cell death or senescence. How this ‘decision’ between repair and death is made has been a bit of a puzzle, but recent work by Hu et al.1 , now shows that a long non-coding RNA (lncRNA), GUARDIN, may provide the answer.
The DNA damage response (DDR)
Simply put, the DNA damage response is a signalling cascade. It involves proteins that recognise the damage and signal to other proteins that then recruit other proteins, amplifying the signal, that then activate effector proteins that may fix the damage, commit the cell to death or irreversibly send the cell into a senescent state…2. For more detail, read this http://www.nature.com/articles/nature08467.
Importantly, regarding the roles of GUARDIN, is the DDR to double stranded breaks (DSBs). There are two main pathways involved in repairing DSBs – non-homologous end joining (NHEJ), a frequent method that effectively just fuses DNA ends back together again and homologous recombination (HR), a more precise repair mechanism that uses a DNA template to correctly fix the damage. A key protein in HR is BRCA1, a protein that is commonly defective in breast cancers.
Downstream of these different signalling pathways is p53. By regulating other proteins, p53 can halt the cell during its progression through the cell cycle, allowing time for the DNA damage to be fixed. However, if the damage cannot be fixed, resulting in persistent activation of the DDR, cell death or senescence may ensue 2.
Understanding these different pathways and how they are regulated is of great interest since these pathways are often defected in cancers. As a key player, this has made p53 a pretty famous protein. Due to this, p53 has been very well studied, racking up a wealthy number of citations. If there were A-list proteins, p53 would be one of them. Anyway, as a transcription factor, p53 controls the expression of many genes. Many of these genes encode proteins, but many more recently characterised genes include long non-coding RNAs (lncRNAs). LncRNAs are RNA transcripts with a range of functions that are not translated to form proteins 3. Hu et al. have recently characterised a lncRNA regulated by p53 they termed GUARDIN. When GUARDIN was knocked down in cells, it triggered cell death and senescence, promoting the team to investigate the role of GUARDIN further.
Telomeres and shelterin
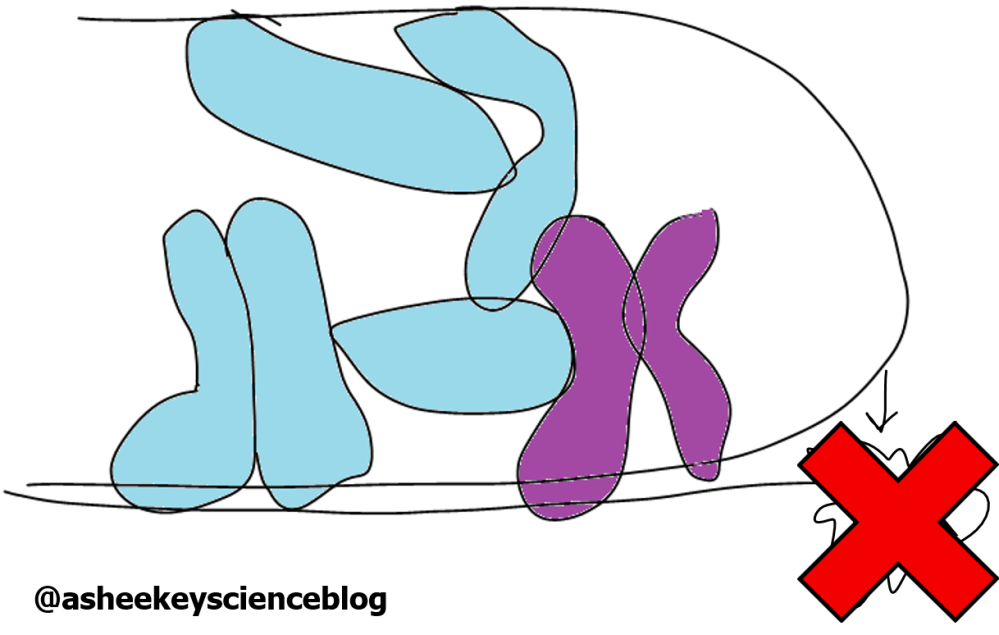
Double strand breaks (DSBs) can activate the DNA damage response (DDR). However, this leaves a bit of a problem. Since DNA is linear, each chromosome has two ends that look identical to DSBs, that if unprotected, can also induce the DDR (Figure 1).

The solution to this problem are the telomeres; hexameric DNA repeats, terminating with one of the DNA strands overhanging, that are wrapped-up in a protein complex called shelterin. Shelterin is composed of six key parts that bind the telomeric repeats hiding the ends of DNA out of sight from the DDR machinery (Figure 2). It’s kind of like you’ve placed a blob of Blu Tack at the end of a string of DNA. Not only does it keep the ends stable, but it also acts as protective barrier. TRF2 (highlighted in purple), expression levels decrease when GUARDIN is downregulated. This is because GUARDIN prevents a TRF2 repressor from repressing. This repressor is a microRNA.
microRNAs (miRNAs)
miRNAs are a class RNAs, but unlike lncRNAs, are shorter and have one main function which is to prevent the translation of certain genes. miR-23a is a miRNA that binds to the mRNA transcript of TRF2, preventing the protein from being produced.
A sponge and a scaffold
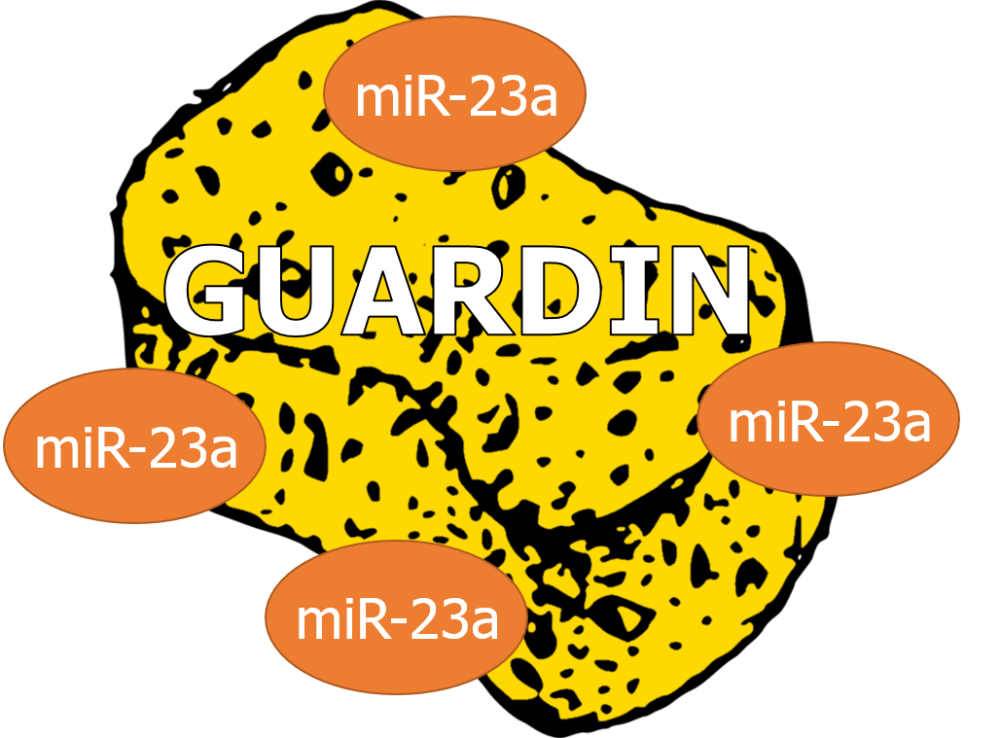
Now, we all know what a sponge is… it soaks up water. The same is true for a miRNA sponge, instead it soaks up miRNAs. GUARDIN contains 8 sequences complementary to the ‘seed’ sequence of miR-23a, suggesting GUARDIN is a pretty good sponge that could potentially bind up to 8 molecules of miR-23a (Figure 3). By sequestering miR-23a, less miR-23a will be around to inhibit the production of TRF2. With more TRF2 around, shelterin complexes will be able to form successfully, preventing activation of the DDR at telomeres.
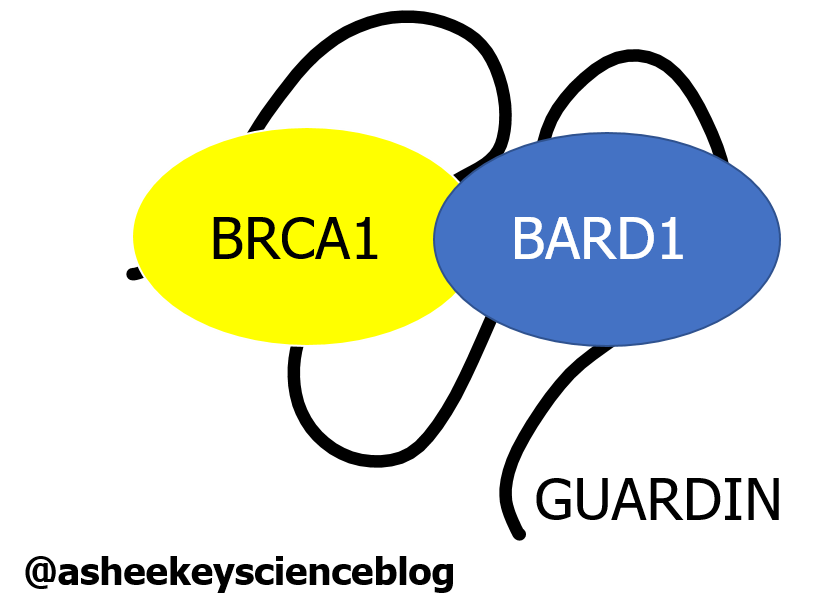
GUARDIN knockdown also resulted in reduction in the levels of BRCA1. GUARDIN knockdown in the presence of a proteasome* inhibitor (MG132), preventing protein degradation, restored BRCA1 levels. Since Hu’s team also showed GUARDIN and BRCA1, along with BARD1 to interact, it suggested that GUARDIN was protecting BRCA1 from degradation. Wahey! Further studies identified binding regions for both BRCA1 and BARD1 on GUARDIN and showed that the three of them formed a complex together. This supports a structural role for GUARDIN to facilitate the heterodimerisation of BRCA1 and BARD1 (Figure 4). BRCA1 and BARD1 are important for repairing DSBs during HR, reinforcing GUARDINs role in guardin’ the genome from damage.
*The proteasome is a protein complex that degrades proteins
So, GUARDIN is a pleiotropic lncRNA that both prevents telomeres from being recognised as a DSB and aids DNA repair. But how does this help? The interesting finding from Hu’s team is the functional relationship between GUARDIN and p53. We know p53 activates GUARDIN, but it seems GUARDIN may modulate the cytotoxic effect of p53 that otherwise triggers cell death or senescence. This is because when GUARDIN was knocked down in a cell without p53, cell survival was not affected. However, re-introduction of p53 induced cell death. Understanding this relationship further may provide rational cancer therapies, with GUARDIN as their target.
This somewhat seems appropriate; Bee Gees – Stayin’ Alive https://www.youtube.com/watch?v=I_izvAbhExY
Further reading
- Hu, W. L. et al. GUARDIN is a p53-responsive long non-coding RNA that is essential for genomic stability. 20, (2018).
- Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
- Mercer, T. R., Dinger, M. E. & Mattick, J. S. Long non-coding RNAs: Insights into functions. Nature Reviews Genetics 10, 155–159 (2009).
One thought on “GUARDIN the genome; a lncRNA unites p53, telomeres and miRNAs”